|
|
FUTURE
ENERGY eNEWS

|
|
NOVEMBER 2011

|
|
|
|
Dear
Subscriber,
We have a great Fifth International
Conference on Future Energy coming up and look forward to
your continued support. It is important to announce that I have been
appointed the Conference Coordinator and Technical and Publications
Chairperson for the SPESIF-COFE5 event coming up February 29
to March 2, 2012. Many of the deadlines now are much more
flexible (send in your abstract for energy, propulsion or
bioenergetics topic for consideration even up until the end of 2011).
It also means that IRI will consider a group of papers, without a
physical presence requirement. We are planning to offer
Webcasting of the event as well as a Remote Presentation capability
for those who cannot make the trip but would like to present over the
Internet. This is a vital and important energy conference folks. All
of the quality papers from COFE4 that were generously this year are now online. View
ALL of the SPESIF2011 and COFE4 papers and download ANY of them for
FREE (pdf): Physics Procedia -
ScienceDirect (c) Elsevier B.V. Elsevier Science has been
contracted for COFE4 and COFE5 to replace the American Institute of
Physics publisher. We feel that Elsevier is better in many
regards and also most importantly, embraces all of the energy topics
that we entertain.
Looking at our top story, IRI
consulted one of the world's experts in Low Energy Nuclear Reactions
(LENR) to get his opinion of the Rossi development. It
is worthwhile to note that Dr. George Miley has equaled the
Rossi performance and is well known in the field for many years.
The Venture Capital article can
be a resource guide for those doing research. The article looking on
the bright side of solar energy after Solyndra is also very valuable
for predicting future energy trends, as well as the last article on
recharging a battery in ten minutes.
For
those bioenergy fans, who keep asking us for more articles on the
topic, we want to emphasize the significance of the #5 article
that announces, "Cancer Craves Killer Free Radicals". If
ever there was a reason to boost your electronic antioxidants
throughout the day, we believe that this is one of the most
convincing. Furthermore, IRI has developed, under Dr. Jacqueline
Panting's direction, "Therapeutic Electronics Antioxidant
Clothing" (patent pending) which answers the concerned senior
citizen's need for daily free radical protection far exceeding pills
or potions. This is because electrons are antioxidants. See http://www.inventionhome.com/InvPortfolio/Portfolio/TV013678/virtual/TV013678.html for more details of the
solution to the free radical disease and aging threat.
Thomas Valone,
PhD, PE Editor
www.IntegrityResearchInstitute.org
|
|
|
|
|
1)
Artificial Photosynthesis to Produce Fuels
|
By Dave
Levitan / November 2011 IEEE Spectrum
http://evworld.com/news.cfm?newsid=25321
|

|
|
Photo: Sun Catalytix
|
If every leaf on the planet can
do it, maybe we can too. Scientists have long tried to mimic
photosynthesis as a way to harness the energy in sunlight
and turn it into a usable fuel, just as plants do. There have been
big technical challenges for just as long, and though scientists are
far from the ultimate goal, two reports published online in the
journal Science yesterday
describe some solutions to the obstacles.
In one
report, a group led by MIT chemistry professor Daniel
Nocera found a new way to use light to split water molecules
into oxygen and hydrogen, which could
then be stored and used as a fuel. Other
groups have had some success with this process before, but there
were always stumbling blocks that would make it hard to scale up or
commercialize, such as extremely acidic or basic conditions,
expensive catalytic materials, or both. However, Nocera's group
managed to get artificial photosynthesis to work using benign
conditions and cheap, abundant materials as catalysts.
Specifically, the team joined a
commercially available triple-junction solar
cell to two catalysts: cobalt-borate for splitting the water
molecule and a nickel-molybdenum-zinc alloy to form the hydrogen gas.
The water-splitting reaction achieved a sunlight-to-fuel conversion
of 4.7 percent in one incarnation of the device and 2.5 percent in
another. The difference between the two was that the more - efficient
device housed the hydrogen-generating alloy on a mesh wired to the
solar cell. The less efficient version was wireless, and the alloy
was instead deposited onto the stainless-steel back of the solar
cell.
It is the wireless possibility,
where the entire device is self-contained, that researchers say is
most exciting. "Because there are no wires, we are not limited
by the size that the light-absorbing material has to be," says
Steven Reece, a research scientist with Sun
Catalytix (a company cofounded by Nocera) who worked on the
discovery. "We can operate on the micro- or even nanoscale...so
you can imagine micro- or nanoparticles, similar to the cells we've
worked with here, dispersed in a solution." The researchers say
they are still deciding what size the final product should
be-anywhere from a small, leaf-sized stand-alone system that might be
able to power an individual home to a much larger system that could
benefit from economies of scale. Whatever size they decide on, the
researchers believe such devices could help provide power in poor
areas that lack consistent sources of electricity.
"As the inputs are light
and water, and the output is fuel, one can certainly see the
applicability of something like that to the developing world,"
says Thomas Jarvi, chief technology officer at Sun Catalytix.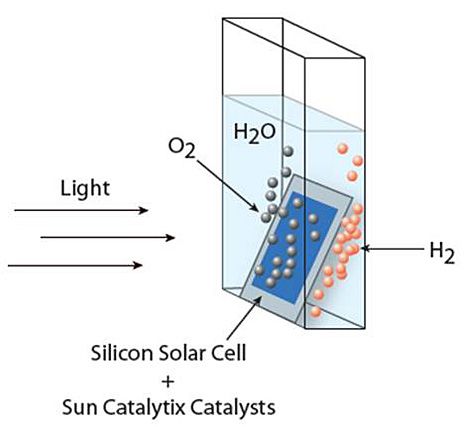
Jarvi says the company expects
to be able to bring the device to the point where a kilogram of
hydrogen could be produced for about US $3. Given that a gallon of
gasoline contains about the same amount of energy as 1 kg of
hydrogen, as long as gas prices stay north of $3 per gallon, this
would make a cost-effective fuel source.
Daniel
Gamelin, a professor of chemistry at the University of Washington
who works on related topics but was not involved with the new study,
says the MIT and Sun Catalytix work represents an "impressive
accomplishment." However, he says, it remains to be seen whether
silicon is really the most desirable material to use, noting that
something less susceptible to degrading by oxygen may be a better
option.
"For these specific
devices, there remain open questions about their long-term
stability," Gamelin says. "And their efficiencies would
still need to be increased substantially to be commercially viable.
But there is obviously potential for improvement on both fronts. In the
bigger scheme, [this research] marks important progress toward the
development of truly practical solar hydrogen technologies."
The
other report, published simultaneously with the hydrogen
producer, demonstrated a different type of advance-a step toward using
sunlight to recycle carbon dioxide. In the natural world, the
sun's energy extracts electrons from a water molecule, which then
reduce CO2 into fuel (in plants, the fuel takes the form
of carbohydrates). University
of Illinois graduate
student Brian Rosen and other scientists were able to invent a device
that electroreduced CO2 to carbon monoxide at a lower
voltage than previously achieved. The high voltages usually required
have been a primary stumbling block in CO2 electroreduction
in the past. Rosen's group brought the voltage down by using a
combination of a silver cathode and an ionic liquid electrolyte that
presumably stabilized the CO2 anion. And according to Rich
Masel, who led the research and is CEO of Dioxide
Materials, a company working on CO2 electroreduction
with the University of Illinois, this piece of the photosynthetic
process could eventually lead to a way to turn captured CO2
into "syngas"-a mixture used in the petrochemical industry
to make gasoline and other fuels.
The experiment "shows that
one can make syngas efficiently from any source of electricity,"
Masel says. However, large-scale versions of the device probably
won't be demonstrated until 2018. "Presently we have
demonstrated the process on the 1-centimeter-squared scale. We need
to go to the million cm2 to make significant amounts of
gasoline."
Work on artificial
photosynthesis has ramped up considerably in recent years. In July
2010, the DOE began funding a Joint
Center for Artificial Photosynthesis to the tune
of $122 million over five years as part of its Energy Innovation Hubs
program; it is led by Caltech professor of chemistry Nate Lewis. The
center, with close to 200 members in universities and national
laboratories across California, aims to build on nature's
photosynthetic design, bridging all the disciplines required, from
chemical engineering to applied physics.
In an interview earlier this
year, Lewis told Spectrum that progress is certainly being
made, but it isn't clear yet if the right combination of catalysts
and light absorbers and everything else that goes into practical
artificial photosynthetic devices has been found.
"We're seeing light in the
tunnel," he said. "We don't know where the end of the
tunnel is. It's a curved tunnel."
|
|
2) PolyPlus Lithium/Water Battery
Could Be "Game Changer"
|
EV World NewsWire, March 3, 2011, http://evworld.com/news.cfm?newsid=25321
Ed. Note: This PolyPlus
invention was also voted one of the "Best 50 Inventions" of
the year in Time magazine. - TV
Batteries made of lithium and
seawater (or just plain tap water for that matter) could be on their
way to a marine market near you.
That's courtesy of a technology made by a 11-year-old company called
PolyPlus and various partnerships, which hails out of Lawrence Berkeley Labs and has
a grant from the Department of Energy's high risk, early-stage ARPA-E
program. At the
annual ARPA-E Summit this week, PolyPlus was highlighted as a
potential game-changer by ARPA-E Director Arun Majumdar, and I got a
chance to sit down with PolyPlus CTO Steven Visco on Monday.
The chemistry
almost sounds like that of science fiction, but Visco told me in an
interview that he thinks the company's water battery could get to
market in two years time, and says the company is just starting the
process of producing a water battery pilot production line now. The
water battery isn't even the end goal for PolyPlus; the company is
developing a non-rechargeable lithium-air and a rechargeable
lithium-air battery, which is the most difficult of the three to
manufacture and for which it received the ARPA-E grant.

Here's how the
water battery works: An encapsulant encloses the lithium,
completely separating it from the water, but still enabling a charge.
That's crucial because lithium and water react rather shockingly
(Visco showed me videos of lithium essentially dissolving in water).
Visco says it
was a Eureka moment when he realized the battery worked, using a
membrane from a third party in Japan and the
company's own three-layer system, and was stable in 2003.
"Cycling lithium and water was absolutely unheard of," and
after that, the company went "dead silent," says Visco, and
turned to filing patent after patent.
A water
battery can achieve awe-inspiring energy densities (the amount of
energy that can be stored in a battery of a given size) of 1,300
wh/kg (for small batches), and potentially 1,500 wh/kg at larger
scale production. For comparison, standard lithium-ion batteries have
closer to 200 wh/kg to 400 wh/kg. That means a water battery can last
a very long time. Picture a battery used for a device on the outside
of a ship, or an underwater unmanned vessel that needs power (hello,
DOD), that can just keep going and going
The water
battery also doesn't have to carry the positive electrode, or the
water, inside it. PolyPlus' water battery has an open system where
the water of the surroundings connects with the lithium. That means
the battery could be more simple and lower cost to produce.
All in
all, Visco thinks the marine battery market could be half a
billion dollars. That could be overambitious, as many of the
applications we discussed are early-stage themselves. But a battery
expert source I talked to about PolyPlus' water battery thought the
device was well on its way and could be a big hit for the company.
The
rechargeable lithium-air battery, for which it received the ARPA-E
grant, could be considerable harder. Though the dream is even bigger:
a battery that one day could make electric vehicles with ranges from
300 to 500 miles. If PolyPlus gets there, it will be at least five
years away, and perhaps two decades before car markers start using
these types of batteries for EVs. It took an innovative car company
like Tesla that long to put standardized lithium-ion batteries into
EVs.
Still, you
have to wonder why PolyPlus hasn't moved into manufacturing before
this. Visco told me the company doesn't want to be just a licensing
company, but wants to be manufacturer and is in the process of
raising funds from VCs and strategic investors right now. When the
funding round are closed, hopefully, the water battery will be on its
way.
RELATED ARTICLES
New Battery Technology Could Provide Large-Scale
Energy Storage for the Grid
Dexter Johnson / Fri, November 25,
2011 IEEE Spectrum
http://spectrum.ieee.org/nanoclast/semiconductors/nanotechnology/new-battery-technology-could-provide-largescale-energy-storage-for-the-grid
I, like many
others, have been following
the work being done by Yi Cui at Stanford University
in improving battery technology.
Cui's work has
often aimed at improving Li-ion battery technology, much in the same
way researchers
at Northwestern University recently have done in getting a
silicon-graphene sandwich to act as a more effective anode.
But in his most
recent research he has abandoned the use of lithium ions and
replaced them with either sodium or potassium ions for his new
battery technology.
The result is
a battery that Cui and his colleagues claim is able to retain 83% of
its charge after 40,000 cycles, which compares more than favorably to
Li-ion batteries of 1,000 cycles.
The
researchers have been able to develop a cathode material that they
can essentially mix in a flask by combining iron with cyanide and
then replacing half of the iron with copper then making crystalline
nanoparticles from the compound.
There is a
weight penalty with this battery technology, which means that it will
not be likely powering any laptops or electric vehicles. However, it
may be the perfect fit for large-scale energy storage on the
electrical grid.
"At a
rate of several cycles per day, this electrode would have a good 30
years of useful life on the electrical grid," said Colin
Wessells, a graduate student in materials science and engineering who
is the lead author of a paper describing the research, published this
week in Nature
Communications.
"That is
a breakthrough in performance - a battery that will keep running for
tens of thousands of cycles and never fail," said Cui, who in
this case is Wessell's adviser and a coauthor of the paper.
But all is not
resolved as of yet. While the researchers have developed this 'new
chemistry' for the battery, they only have the high-power cathode at
this point, so they still need to develop an anode.
Nonetheless
the researchers are confident they will develop a material for the
anode. If they manage to get that sorted, they may have developed an
economical battery for storing energy from solar and wind power so as
to avoid sharp drop offs in electricity in the grid.
ADDICTED TO POWER: USING
TECHNOLOGY TO BUILD A BETTER BATTERY
Ben Bajarin
TIME.com
http://techland.time.com/2011/11/21/addicted-to-power-using-technology-to-build-better-batteries/
When walking
through an airport, have you ever tried to find an outlet for your
computer or to charge your phone only to realize that every last
outlet is being used? I have this experience often since I travel for
business quite a bit. The same is true of my house. There never seems
to be enough plugs to charge all my gadgets. Then again, I have too
many gadgets.
Whenever I
have this experience, I am reminded of the sad state of battery
technology for our mobile devices. The constant need to charge our gadgets
is about as irritating to me as having to put gas in my car.
Charging, like having to get gas, is an irritating task. It makes me
feel like somehow my freedom is restricted-and in a way, it is.
(MORE: TV
Needs to Be Reinvented)
On Twitter
this week, Bill
Gates put out a call for the creation of new and better
renewable energy sources. He also shared
a stat I thought was interesting: All the batteries on Earth
store just 10 minutes worth of world electricity needs.
Unfortunately,
battery technology is a limited science. We don't have the luxury of
having our battery technology follow the pace of innovation or
technological advancements like we do with other technologies. This
is going to be a limiting factor for the foreseeable future, too.
Technology innovation
is not bad, of course. It's good and encouraging. But having said
that, our issues with short battery life are partially our fault. The
market's desire for thinner PCs, smartphones, and tablets with
brighter screens and faster processors all require making tradeoffs
that impact battery life. Innovation isn't bad, as I said, but the
reality is that our desire for innovative electronics is hampered by
the limited science of our current battery technology.
So what can be
done about it? Is there hope, or are we doomed to need to recharge
all our gadgets on a daily basis? There are several things happening
that I want to highlight, along with emphasizing that more still
needs to be done.
The first is
advancements in microprocessors. The brains that power our
electronics have come a long way. Every company making
microprocessors for PCs, tablets, smartphones and any other mobile
technology we dream up is working on creating more power efficient
processors. The goal is to create processors that are still powerful,
but don't require more power themselves, which drains battery life.
This is important because as we demand more processing power in our
devices to do things like run our apps, play media-rich games and
browse multimedia-filled web pages, we need faster CPUs.
(MORE: Do
Windows 8 Tablets Stand a Chance?)
If you follow
the technology industry, you're familiar with a term called "Moore's
Law." One of Intel's founders, Gordon E. Moore stated that the
number of transistors which could be placed on a single chip would
double every 18 months. Note that this does not mean processing
performance necessarily doubles every 18 months-only the number of
transistors.
There is a key
observation, however, for Moore's
Law and mobile devices. Moore's Law not only makes it possible to
double transistors every 18 months, but it also paves the way for
chips to become smaller and, in turn, require less power. This is why
companies like Intel and AMD are racing to create new processor
architectures on an annual cadence. With each new generation, we can
have roughly the same computing power, but with smaller processors
which require less power. Key advancements by all players in the
silicon space are happening in a way that, over time, will see
significant computing power that requires less battery power to
achieve.
The second
thing that is happening is experimentation around battery technology
itself. As I stated previously, lithium-ion battery technology is a
limited science. People have been trying to achieve breakthroughs
with this technology for some time with little success. However,
Northwestern University recently
released a report and white paper stating that researchers there
had created created an electrode for lithium-ion batteries that
allows the batteries to hold a charge up to 10 times greater than
current technology and can charge 10 times faster than current
batteries.
As with all
early research, it takes time and money to see if these new
technologies could be sustained and produced commercially for the
mass market. This new research out of Northwestern is encouraging,
and I'm hearing of work in other technology labs that are also trying
to create breakthroughs with lithium-ion batteries.
Unfortunately,
making technological advancements in microprocessors, lithium-ion
batteries, and perhaps some new energy source simply takes time. The
important thing is that key work is being done to address our battery
life issues with our devices. So for the foreseeable future we will
still have to fight for outlets at the airport and charge our
smartphones at least once each day. But that's the reality of today;
hopefully not the reality of tomorrow.
(MORE: In
the Future, We Will All Talk to Computers)
Ben Bajarin is
the Director of Consumer Technology Analysis and Research at Creative
Strategies, Inc, a technology industry analysis and
market intelligence firm located in Silicon
Valley.
|
|
3)Advance Could Challenge China's
Solar Dominance
|
Technology
Review, November 21, 2011 By Kevin Bullis
http://www.technologyreview.com/energy/39157/?nlid=nlenrg&nld=2011-11-21
Chinese solar-panel manufacturers dominate the
industry, but a new way of making an exotic type of crystalline
silicon might benefit solar companies outside of China that have
designs that take advantage of the material.
GT Advanced Technologies,
one of world's biggest suppliers of furnaces for turning silicon into
large crystalline cubes that can then be sliced to make
wafers for solar cells, recently announced two advanced technologies
for making crystalline silicon. The new approaches significantly
lower the cost of making high-end crystalline silicon for highly efficient
solar cells.

The first technology, which GT calls Monocast,
can be applied as a retrofit to existing furnaces, making it possible
to produce monocrystalline silicon using the same equipment now used
to make lower quality multicrystalline silicon. It will be available
early next year. Several other
manufacturers are developing similar technology.
It's the second technology, which the company
calls HiCz, that could have a bigger long-term impact. It cuts the
cost of making a type of monocrystalline silicon that is leavened
with trace amounts of phosphorous, which further boosts a panel's
efficiency. This type of silicon is currently used in only 10 percent
of solar panels because of its high cost, but could gain a bigger
share of the market as a result of the cost savings (up to 40
percent) from GT's technology. The technology will be available
next year.
A standard solar panel, made of multicrystalline
silicon, might generate 230 watts in full sunlight. A panel the same
size made of monocrystalline silicon could generate 245 watts. But
phosphorous-doped monocrystalline silicon (also called n-type
monocrystalline) enables a type of solar panel that generates 320
watts, a huge leap in performance.
Most Chinese solar manufacturers have focused on
multicrystalline silicon solar panels. Companies such as U.S.-based
Sunpower have focused on the advanced monocrystalline panels, and
have designed cells to exploit its properties. Such companies will
benefit as the HiCz technique developed by GT Advanced Technologies
becomes more common.
"There's a potential shift in the
market," says Vikram Singh, general manager for the photovoltaic
division at GT Advanced Technologies. He says some western companies
could become more competitive because they have technologies to take
advantage of the materials.
Several other companies are developing
technologies similar to Monocast, including solar-panel makers in China,
such as Suntech and the Dutch equipment maker ALD.
The HiCz technology can be considered the next
step on the way to higher-efficiency solar cells. It can be used to
make monocrystalline silicon, even the phosphorous-doped type, for
about the same cost as the Monocast technology. HiCz could allow a
leap from cells that convert 16 to 18 percent of the energy in
sunlight into electricity to ones that can convert 22 to 24 percent,
thus decreasing the cost per watt of solar power. But it can't be
retrofitted to existing equipment, which could slow its adoption.
The conventional way to make monocrystalline
silicon is to introduce a seed crystal into a pool of molten silicon
and slowly draw it out-as you do, it forms a large tube-shaped chunk
of silicon called a boule, in which all of the atoms are lined up in
the same orientation. This is usually done in a batch process, but
the HiCz process makes it possible to continuously feed in raw
silicon to the melt, along with whatever trace elements are needed to
give it the desired electronic properties. The continuous process is
more productive, which means fewer machines are needed, reducing
costs. It also produces high yields when introducing materials
including trace elements such as gallium and phosphorous. GT
estimates the process can reduce the costs of making monocrystalline
solar by between 20 and 40 percent.
RELATED ARTICLE
US Eyes Deal Outside WTO on
China's Green Subsidies - Solar Panels
Bridges Weekly Trade News Digest
2 November 2011 Vol. 15 No. 37 , Inter Centre for Trade and Sustainable
Development, http://ictsd.org/i/news/bridgesweekly/117357/
The US will take advantage of
several high-level meetings in Asia this month to address barriers to
trade in environmental goods and services (EGS). Tensions between
Washington and Beijing have been high in recent months as US
lawmakers and manufacturers have increasingly sought action against
China's green subsidies.
US Trade Representative (USTR)
Ron Kirk told a business group last week that he would push for a
voluntary tariff binding of five percent on a "basket of
issues" relating to green technologies, facilitating trade
between a number of nations competing for a stake in the new energy
sector.
The US will raise the issue with
China and other Asia-Pacific Economic Cooperation (APEC) partners at
a meeting of the regional body in Honolulu next week. The US will
also have the opportunity to discuss the arrangement one-on-one with
China shortly after the APEC summit at the US-China Joint Commission
on Commerce and Trade, and in meetings on the sidelines of APEC with
eight additional members that are involved in the ongoing
Trans-Pacific Partnership talks.
While Kirk says he has the support of Australia,
New Zealand and others, a trade diplomat told Reuters that China
prefers to leave the matter to the WTO. Keeping the negotiations in
Geneva would allow China to cut a tariff deal in exchange for trade
concessions from other WTO members, while also preventing the deal
from moving forward on a voluntary basis. If the tariff bindings are
to become WTO-enforceable, observers suggest that EGS negotiations
could become far more complex.
|
|
4) Small Nukes Get
Boost
|
By Kevin
Bullis http://www.technologyreview.com/energy/38897/?nlid=nlenrg&nld=2011-10-24
The large
engineering and construction firm Fluor has taken a majority stake in
NuScale Power, a startup that has been developing small, modular
nuclear reactors. The investment effectively rescues NuScale, which
had been near financial collapse after its biggest investor was
indicted by the U.S. Securities and Exchange Commission for violating
regulations.
The deal with
Fluor will allow NuScale to continue its efforts to license its power
plant design with the U.S. Nuclear Regulatory Commission, with the
goal of having the first one up and running by 2020. Fluor's
engineers will help with the certification work, and the company
eventually plans to engineer and build NuScale's power plants.
The investment
by Fluor is a vote of confidence in small modular nuclear reactors.
These reactors generate 300 megawatts or less, about a third of what
conventional nuclear reactors generate, and are designed to be safer
and easier to manufacture. The technology has been gaining attention
in recent years as high costs and safety concerns, such as those
kindled by the nuclear accident at Fukushima, have hurt the prospects
of large, conventional nuclear power plants. At the same time,
organizations such as the International Atomic Energy Agency are
anticipating a large market for small nuclear reactors in poor
countries and in rural areas that don't have the infrastructure or
demand to accommodate conventional large reactors.

Other major
engineering and construction companies in the nuclear industry have
recently shown support for small modular reactors, including Bechtel
and Babcock & Wilcox, which this summer
announced a partnership with the Tennessee Valley Authority to
work toward building six of Babcock and Wilcox's small mPower
reactors. Worldwide, dozens of designs being developed, including
efforts in Japan, Korea, China, Russia, and Argentina. U.S.
Energy Secretary Steven Chu has made development and licensing of
small modular reactors a focus for the U.S.Dept of Energy
The NuScale
reactor design is based on technology developed by the DOE and Oregon
State University, which was involved in the design and certification
of the new Westinghouse AP1000 power plants that are being built now
in China and at two locations in the United States. The reactor is a
type of light water reactor, one of the most common types of reactors
in use today. NuScale has completed a detailed preliminary design,
and intends to submit a design certification application to the NRC
next year.
NuScale's
reactors are designed to generate 40 megawatts each, compared to over
1,000 megawatts for conventional reactors. They can be linked
together on site to generate larger amounts of electricity.
Traditionally, nuclear power plants have been built large to take
advantage of economies of scale. But the large size of the projects
leads to long construction times, and delays and cost overruns are
common, heightening the risk for investors and increasing financing
costs.
Smaller
reactors, which can be built in factories rather than assembled on
site, could be faster to build, lowering financing costs. The designs
can also be simpler, and thus cheaper than conventional nuclear power
plants, since the smaller reactors require lower pressures, for
example, and their small size makes it practical to combine multiple
elements into one containment vessel. Some experts have calculated
that costs per megawatt could be comparable to large nuclear
reactors, but no one really knows because no small, modular
commercial nuclear power plants have been built yet.
Even if costs per megawatt
prove higher than with conventional plants, their small size might
make them attractive in areas that lack the power lines and other
infrastructure needed to distribute power from a large reactor, and
that may not immediately have demand for the full power output of a
large reactor. The modular design could allow utilities to gradually
add more reactors as demand increases. Several rural electric cooperatives
in the United States have expressed interest in using NuScale's small
nuclear reactors to replace aging coal plants-the small size of the
plants would eliminate the need to upgrade existing transmission
lines. Critics of small nuclear reactors, such as the Union of
Concerned Scientists, say that large numbers of small reactors could
be more difficult to manage during an accident, and could pose
greater risk of nuclear materials falling into the hands of
terrorists or rogue states.
|
|
5) Red Laser and
Green Tea Attack Alzheimer's
|
New Scientist, November 2011
http://www.newscientist.com/article/mg21228374.600
IT MAY sound like a strange brew,
but green tea and red light could provide a novel treatment for
Alzheimer's disease. Together, the two can destroy the rogue
"plaques" that crowd the brains of people with the disease.
The light makes it easier for the green-tea extract to get to work on
the plaques.
|

|
|
Brain showing Alzheimer's disease. Photo
courtesy Science Daily
|
Andrei Sommer at the University
of Ulm in Germany, and colleagues, have previously used red light
with a wavelength of 670 nanometres to transport cancer drugs into
cells. The laser light pushes water out of the cells and when the
laser is switched off, the cells "suck in" water and any
other molecules, including drugs, from their surroundings.
Now, Sommer's team have found
that the same technique can be used to destroy the beta-amyloid
plaques in Alzheimer's. These plaques consist of abnormally folded peptides,
and are thought to disrupt communication between nerve cells, leading
to loss of memory and other symptoms.
The team bathed brain cells
containing beta-amyloid in epigallocatechin gallate (EGCG) - a
green-tea extract known to have beta-amyloid inhibiting properties -
at the same time as stimulating the cells with red light.
Beta-amyloid in the cells reduced by around 60 per cent. Shining the
laser light alone onto cells reduced beta-amyloid by around 20 per
cent (Photomedicine and Laser Surgery, DOI: 10.1089/pho.2011.3073).
It can be difficult getting
drugs into the brain, but animal experiments show that the green-tea
extract can penetrate the so-called blood-brain barrier when given
orally together with red light. The light, which can penetrate tissue
and bone, stimulates cell mitochondria to kick-start a process that
increases the barrier's permeability, says Sommer.
There is no reason why other
drugs that attack beta-amyloid could not be delivered to the brain in
the same way, he adds.
"This important research
could form the basis of a potential treatment for Alzheimer's, with
or without complementary drug treatment," says Mario Trelles,
medical director of the Vilafortuny Medical Institute in Cambrils,
Spain.
"The technique described could
help to regulate and even stop the appearance of this disease,"
he adds.
back to table of contents
|
About
Integrity Research Institute
Future Energy eNews is
provided as a public service from Integrity Research Institute, a
Non-Profit dedicated to educating the public on eco-friendly
emerging energy technologies.
FREE copy of the 30 minute DVD
"Progress in Future Energy" is available by sending an
email with "Free DVD" in subject and mailing
address in body.
Your generous support is welcome by making a tax
deductible donation on our secure website
|
|
|
|
|
Save 10%
|
On all purchases from IRI by becoming a member and a free gift when you join and you save 10% on
all conference and workshop fees as well. You will receive
a quarterly mailing with the latest information on eco-friendly
emerging energy technologies. All 2011 IRI members will receive free
copies of Energy magazines and the latest emerging energy technologies
reports.
|
|
|
|
|
|